| Adenine | |
|---|---|
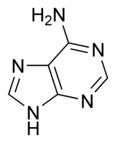
| Chemical name | 9H-Purin-6-amine |
| Alternate name | 6-aminopurine |
| Chemical formula | C5H5N5 |
| Molecular mass | 135.1267 g/mol |
| Melting point | 360 - 365 °C |
| CAS number | 73-24-5 |
| SMILES | NC1=NC=NC2=C1N=CN2 |
 |
|
Adenine is one of the two purine nucleobases used in forming nucleotides of the nucleic acids DNA and RNA. In DNA, adenine binds to thymine via two hydrogen bonds to assist in stabilizing the nucleic acid structures. In RNA, adenine binds to uracil, which is used in the cytoplasm for protein synthesis.
It forms several tautomers, compounds that can be rapidly interconverted and are often considered equivalent. Guanine, a related compounds (also a purine derivative), forms tautomers in the same way, and has more detailed information too.
Adenine forms adenosine, a nucleoside, when attached to ribose, and deoxyadenosine when attached to deoxyribose; it forms adenosine triphosphate (ATP), a nucleotide, when three phosphate groups are added to adenosine. Adenosine triphosphate is used in cellular metabolism as one of the basic methods of transferring chemical energy between chemical reactions.
In older literature, adenine was sometimes called Vitamin B4. However it is no longer considered a true vitamin nor part of Vitamin B.
Some think that, at the origin of life on Earth, the first adenine was formed by the polymerizing of five hydrogen cyanide (HCN) molecules. However, this has been criticized by some chemists [1].
External links
- Computational Chemistry Wiki
- Link page to external chemical sources.




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xxx
216.73.xxx.xxx
 Server Time:
Server Time: